It seemed appropriate to take a look at something that we mentioned a bit last week. If you read last week’s article about Wilhelm Röntgen and thought to yourself “but how do those x-rays come into existence?”, then you’re in luck! The discovery of x-rays opened us up to all kinds of technologies and innovation in the fields of physics, chemistry, medicine, cosmology, and much more. X-rays straddle the line between benign and malignant, and gently fondle the transition from ultraviolet to gamma rays.

Today we’re going to take a look at how x-rays come into being, how we make them, and the physics behind how they let doctors see the object you “fell on.”
The History
Though we normally think about x-rays as being a largely man-made phenomenon, x-rays have been around much much longer than their discovery by man. Natural sources of x-rays include cosmic bodies, radioactive elements, and Superman. Cosmic radiation, which refers to all high-energy radiation coming from space, is caused by interactions in celestial bodies that fling high-energy particles our way. X-rays coming from radioactive materials are the result of certain elements rearranging themselves into more stable molecules; all that shuffling around can create radiation.
Knowledge of x-rays began when Wilhelm Röntgen discovered them in his lab in 1901. He was working with a cathode ray tube (more on that in a moment) when he noticed that even with the tube sealed in a light-tight box, a cardboard target painted with fluorescent material would glow when he brought it close to the box. After he was sure that he had discovered a new kind of ray, he dubbed the mysterious rays “x-rays” because of their unknown nature. The pictures painted by the rays were different depending on what was in between the box and the target – Wilhelm had discovered that x-rays travel differently through various materials.
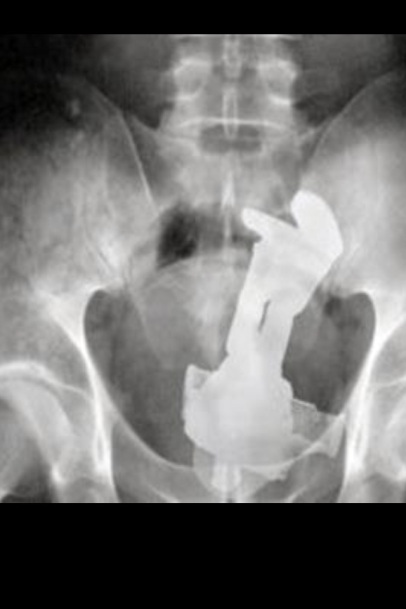
Almost immediately, the potential medical applications became apparent. Now, doctors could take a look into a patient without slicing them open, and medical problems were diagnosed easily and quickly. Modern CT scanners work in much the same way, but by taking x-ray photographs in “slices”, they can make a 3D of model a patient’s body by combining all of these slices together – just like how putting actual slices of a patient can model his body, but with much less screaming involved.
In recent years, x-rays have been observed from celestial bodies, and telescopes tuned to detect x-rays have captured images of objects from the cosmos. Earth’s atmosphere makes it difficult to detect them from the ground, so these telescopes are often placed at high altitude or hitch rides on satellites in order to obtain a clear image.

X-rays are used in crystallography, spectroscopy, and a bunch of other types of scopies and graphies. Let’s not get into it. Instead, let’s see how we conjure up this radiation.
The How
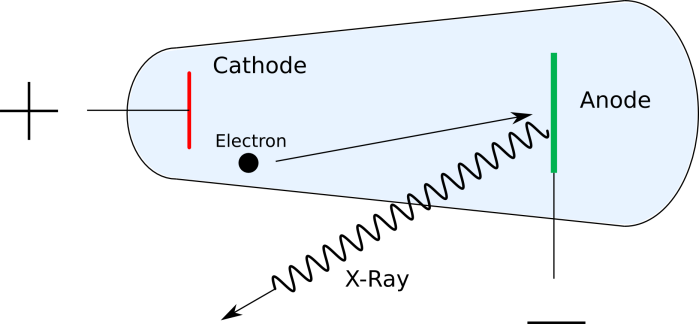
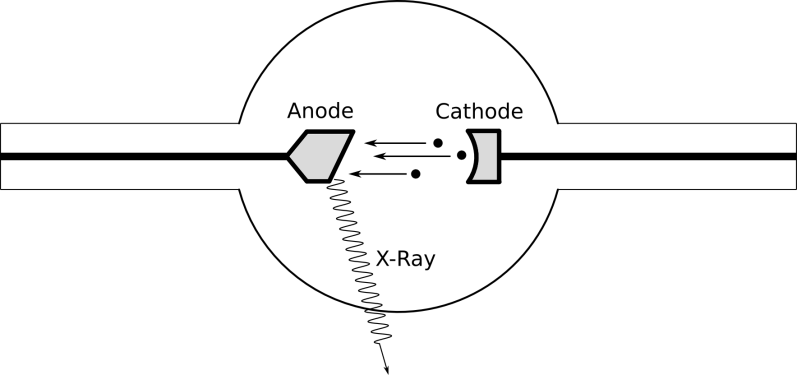
The first x-rays were produced accidentally with the use of Crookes tubes, a type of cathode ray tube. These are hollow glass tubes filled with gas at low pressure. At one end of the tube, a negatively charged metal contact called a cathode, and at the other end, a positively charged metal contact called an anode. When a large voltage is applied to the contacts, energy is imparted to the atoms in the gas and cause them to shed electrons in a process called ionization. The negatively charged electrons are pushed along by the electric field created by the electrodes, and go flying off toward the anode, to which they are attracted. When the electrons reach the anode, x-rays are created by the collisions between the fast-moving electrons and the surface of the anode.
Later advancements led to the invention of the Coolidge tube, which is the most commonly used type of x-ray tube now. They’re similar to Crookes tubes, but rather than using ionizing gas to get electrons, Coolidge tubes use thermionic emission to produce electrons. You can read more about it here, but in short, thermionic emission is the process by which electrons are emitted from materials when they’re heated to high temperatures. Instead of filling the tube with gas, these new tubes are vacuum tubes and contain no gas within the glass enclosure. Electrons still travel from cathode to anode and produce x-rays upon striking the anode surface. Tilting the anode to one side causes x-rays to be emitted in a specific direction, and allows the tube to be aimed.
Before we get to the physics I just glossed over there, let’s consider what happens when we squishy humans get in the way of x-rays.
As we know, x-ray images show up because the rays travel through some materials better than they do others. Elements with higher atomic numbers do a better job of absorbing x-rays, while others allow them to pass through. For this reason, the calcium in bones blocks more radiation than the tissue surrounding it, creating an image that clearly shows the structure of the human body.
What about the danger associated with x-rays? It’s well known that excess exposure to radiation can cause cancer. To measure exposure to radiation, we can use the SI unit of , or Coulombs over kilograms, measuring the amount of radiation needed to create one Coulomb of charge for each kilogram of mass interacting with the radiation. That’s not very intuitive, so we just normally use rem or Sv to measure exposure to all types of radiation. Röntgen equivalent man (rem) is the traditional unit of measure and sievert (Sv) is the SI unit for equivalent dose, or the amount of radiation absorbed by the human body. Just milling about your daily lives exposes you to about 4 milli-sieverts (
Sv) every year. For reference, getting an x-ray done on your arm is about 1 micro-sievert (
Sv) , while a chest CT-scan is about 7 (
Sv). Taking a flight from New York to LA exposes you to 40
Sv (at high altitude, your exposure to cosmic radiation rises because there is less atmosphere above to protect you). It’s fairly safe to absorb small amounts of radiation over time, but sudden exposure to radiation poses a safety risk. Radiation starts getting dangerous at around 1 Sv, which if absorbed all at once could lead to radiation sickness. It’s estimated that standing right next to the Chernobyl reactor for 10 minutes right after the meltdown would expose you to 50 Sv. You’re dead, kiddo, because exposure to only 8 Sv would prove fatal regardless of whether you are able to receive treatment or not.
The reason that dentists put that reassuringly heavy blanket on your chest while you’re get a dental x-ray done? Heavy elements like lead can be used as shielding to protect patients from exposure to x-rays. Dental x-rays for instance, are very tightly focused so as to not affect the rest of your body, but the lead blanket is placed there just to minimize exposure. Even then, dental x-rays only expose you to about 5 Sv of radiation. Why leave the room then? They probably have to give a whole bunch of x-rays every day, so the less time they spend hanging out with running x-ray machines, the better.
Okay, moving on from the doom and gloom and onto everyone’s favorite part!
The Physics
It might seem a little scary that we could produce potentially dangerous radiation with just the flick of a switch, but the scary part is that it doesn’t even take that much power to make it go. When I was doing my undergrad, we ran experiments with an x-ray tube that just plugged into the wall. I wouldn’t be surprised if it consumed less power than the space heater I use to keep my feet warm while I write this blog.
Let’s take it back to the x-ray tubes. We’ve got an evacuated glass tube with some metal electrodes in them, and electrons streaming from one side of the tube to other at high speed. What’s of interest here is what happens when those electrons from the cathode reach their target on the anode side. When these electrons collide with the anode surface at the end of the tube, two processes can occur: x-ray generation from Bremsstrahlung, and the production of characteristic x-rays. The first one first.
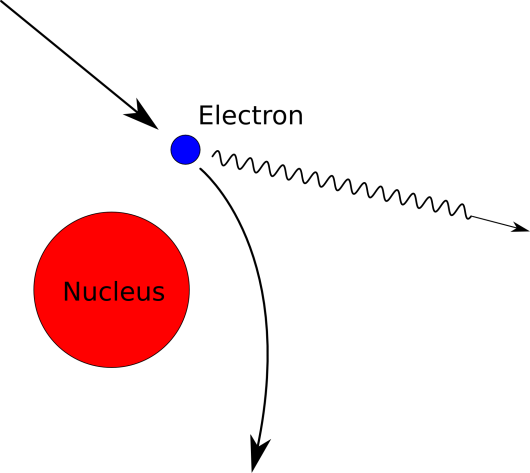
Bremsstrahlung (German for “braking radiation”, which demands that you pronounce the word with vigor) comes about when one charged particle passes close to another. In our case, the negatively charged electron is zooming by a positively charged proton in the nucleus of the atom. The electron will have a tendency to slow down due to being attracted by the proton, but it can’t just slow down for free. The cost of braking is that the electron has to lose energy somehow because of the law of conservation of energy (you can’t just destroy energy all willy-nilly). So, that extra energy pops out of the electron in the form of a photon – our x-ray photon. Yes, these are the same photons that make up light since, if you haven’t figured it out by now, x-rays are just the same as light but with very high frequency (or short wavelength, if you prefer to jive that way).
A relationship called the Duane-Hunt law shows the connection between the wavelength of the resulting photon and the voltage that we pump into the x-ray tube.
,where is the wavelength of the x-ray,
is Planck’s constant,
is the speed of light, and
is the voltage that the electron passes through in the x-ray tube, measured in kV.
As we increase the voltage in the x-ray tube, the minimum wavelength gets smaller. This corresponds to higher energy rays by a quantum mechanics equation that describes the energy of a photon depending on its frequency, or inverse wavelength.
,where is the frequency of the photon.
Presumably, we could just keep pumping up the power to produce photons that have energies in the gamma ray range, but all those collisions produce heat, and we’re somewhat limited by how much heat we can pump away from the x-ray tube before things start to melt.
Next up, characteristic x-rays! Characteristic x-rays are produced when the fast flying electrons actually collide with and knock off an electron from an atom on the anode target. Electrons are arranged in levels called energy levels in the atom, and if the incoming electrons manage to knock away an electron close to the nucleus, then another electron from a higher energy level will have to fall into its place. Because electrons in higher energy levels have…well…more energy, they have to lose that excess energy when they fall into a lower level. What happens to that extra energy? You guessed it; it becomes a photon. What’s interesting about characteristic x-rays is that the energy of the x-ray that is produced is very specific to the target material. It’s like a fingerprint for every material, which is why these x-rays are called characteristic x-rays. They can tell us about what the target is made of, and it’s all dependent on the electronic structure of each element. Measuring the energy of the x-rays coming from x-ray tubes will give us a picture that looks something like this:

The x-rays emitted are able to zip right through you, and paint a picture of what’s inside of us. Thank them the next time you’ve got a tummy ache, and a medical professional doesn’t have to guess what’s ailing you, or take you into the operating room just to tell you that everything is fine.