I love ice cream. I love fried stuff. When the marriage of two culinary genius come together, they create the perfect blend of gluttonous joy. Deep fried ice cream is deeply engrained in my childhood memories; we used to order it all the time from our local sushi joint who only had two flavors: green tea ice cream and mango ice cream. Not exactly the kinds of flavors every small child yearns for, but it was delicious nonetheless. As an adult, I can fully appreciate the blend of flavors, textures, and temperatures associated with this dessert, as well as a newfound appreciation for how it’s made, and why it’s even possible. Here, we’ll take a look at the origins, recipe, and underlying physics of this delicious and impossible dish. Get ‘yer spoons ready, folks!
The History
Like almost all food items, the history of deep fried ice cream is highly contested – some say it started off in Chicago or Philadelphia during the 19th century. Others claim it originated in Japanese restaurants. Whatever the case, the description of the dish is the same: a ball of frozen ice cream rolled in something crispy, then dipped in boiling lard, butter, or oil. Maud C. Cook, in her 1897 book titled Breakfast Dinner and Supper, or What to Eat and How to Prepare It, said “It is pronounced delicious.” Yes, Maud. Yes it is.

In its modern form commonly found in Asian restaurants, deep fried ice cream is normally coated in tempura batter. If you’ve never had tempura before, it’s a light, fluffy, and crispy batter usually reserved for frying dignified foods like seafood and vegetables. Somewhere along the way, a daring entrepreneur thought “Hey, Americans like fried food! And they love ice cream!”, and the bastard child of that combination was born.
The How
You might be saying, “But I thought that ice cream melts when it gets warm!”, and you’d be right. Deep fried ice cream works for two reasons – the temperature of the ice cream, and the application of pound cake or a similar bread/cake-like food. The ice cream is first scooped into balls, then frozen and kept at a temperature well below normal ice-cream-keeping-temperatures (a technical term). The typical household freezer can get as cold as about , but the blast chillers used in restaurants can reach temperatures well below that – around
. This ensures that the temperature stays well below the
melting point of the ice cream when heated.
After that, pound cake is packed around the ice cream (not too soft, not too firm) to provide it with thermal insulation against the boiling hot oil it’s going to be tossed into. The whole package is then placed back in the freezer for a little extra insurance. When it’s ready to cook, it’s coated with tempura batter, quickly deep fried until GBD (golden brown and delicious, courtesy of Alton Brown), served – usually with a healthy dose of whipped cream, powdered sugar, and chocolate sauce, and quickly devoured in a fit of glee.
But how well does the pound cake work in keeping things chilly on the inside?
The Physics
Let’s talk about heat transfer.
As you probably know, heat is the spontaneous transfer of thermal energy from a region of high temperature to low temperature. This can happen in a few ways: conduction, convection, and radiation. Conduction is the transfer of energy from one medium to another by physical contact. Convection relies on the motion of a fluid (air is a fluid too!) to carry energy away from the source. Radiation involves the transfer of energy via electromagnetic radiation generated by anything with a temperature above absolute zero (so basically, everything). The intricacies of these things are for talks for another day, but let’s just talk about conduction, since that’s what we’ll be dealing with here.
First, let’s define temperature. While most know it as a measure of how hot or cold something is, us geeks know it as a measure of the average kinetic motion of the molecules that make up objects. These molecules vibrate, spin, and move around, and when they’re moving a lot, an object has high temperature. When you put two objects in contact with each other, the one with fast-moving particles (high temperature) is going to impart some energy on the cooler object when those particles slam together. Passing this energy along is heat conduction – the transfer of thermal energy via direct contact. We can illustrate this concept with a sweet little equation called Fourier’s Law.
Jean Baptiste Joseph Fourier was a French mathematician and physicist working in the mid 18th century. He was initially studying to become a priest, but at 21 decided “nah” and went on to study his true passion – mathematics. Fourier eventually ended up as Napoleon’s scientific advisor while he (Napoleon, not Fourier) was invading Egypt and was appointed to be the governor of Lower (Northern) Egypt. While there, he and Napoleon along with several other big names in math founded the Cairo Institute, dedicated to organizing the scientific work done in Egypt. After leaving Egypt, Fourier began his work on heat laws, as well as his proposal that mathematical functions can be described with a series of trigonometric functions….more on that another day.

Aaaaanyway, Fourier’s Law describes the exchange of heat between two sides of a barrier, and is written as:
,where is the heat transferred over time,
is the temperature difference between the two sides of a barrier over its thickness, A is the surface area of the barrier, and
is the thermal conductivity.
It’s all a huge jumble, but basically, you can say that the amount of heat transferred over some barrier in some amount of time will increase if: the surface area of the barrier is larger, the difference in temperature is larger, the thickness of the barrier is smaller, or the thermal conductivity is smaller. Most of that is pretty much common sense – a thick slab of concrete is going to block more heat than a thin slab of concrete, it’s more efficient to have smaller windows in your house, and heat won’t flow between two objects of the same temperature. Thermal conductivity is a measure of how easily a material conducts heat; a material with high thermal conductivity is a good conductor of heat, and one with low thermal conductivity is a good insulator. For instance, let’s consider carbon steel () and aluminum (
). It’s easy to see here that two similarly sized barriers of steel and aluminum conduct heat very differently, and that aluminum is much better at it.
For our deep fried ice cream, Fourier’s law translates thusly: the amount of heat transferred into the ice cream over the cooking period depends on the surface area of the pound cake barrier, temperatures of the ice cream and oil, thickness of the pound cake, and the thermal conductivity of pound cake.
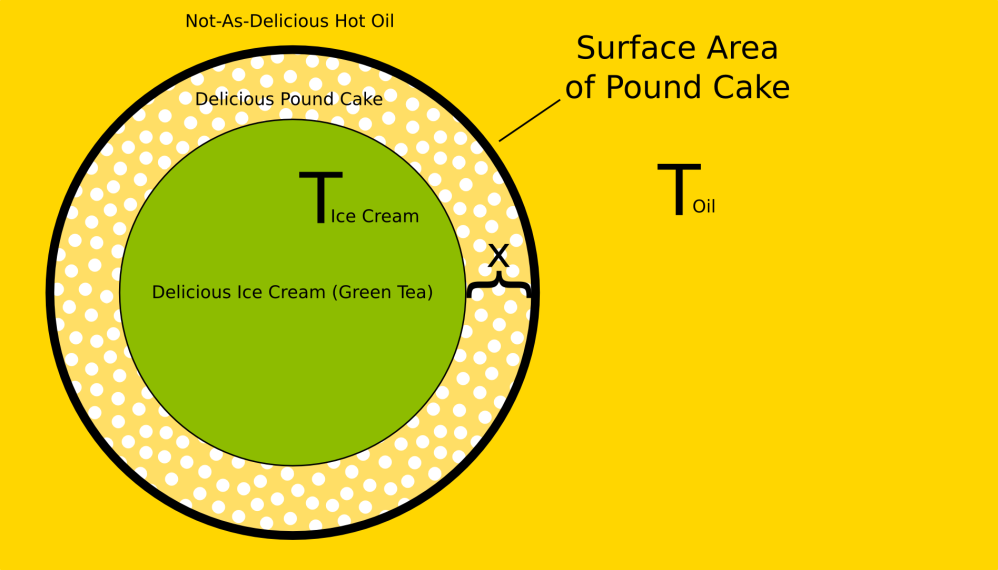 What then, is the thermal conductivity of pound cake?
What then, is the thermal conductivity of pound cake?
Funny you should ask that, because there has actually been a study on the thermal conductivity of baked foods. I’m not making this up! It was conducted at my alma mater, The Ohio State University in 2006 by the Department of Food Science and Technology and tested the thermal conductivity of baked goods as a function of porosity, or how porous the foods are. If you think about it, it really does make sense – the more porous something is, the more air is in it, and the more the thermal conductivity will resemble that of air. Take silica aerogel for instance: this stuff is a solid synthetic ultra-porous material that is composed of about 98% air, yet is extremely strong for its weight, and one of the best solid insulators in the world. It owes its thermal properties to the fact that it’s mostly made of gas, and has a thermal conductivity of around 0.03 – which is almost as low as air.
Our researchers at OSU found that pound cake had a thermal conductivity of 0.110 , on par with lightweight concrete, natural rubber, and most wood varieties. Pound cake’s thermal properties are tied to the fact that it is filled with air, but I wouldn’t recommend packing your walls with it – it won’t perform nearly as well as fiberglass insulation. Likewise, I don’t recommend packing your ice cream with fiberglass insulation – it’ll stay chilly, but won’t taste nearly as sweet, and will certainly taste more itchy.